.gif) VIARTIS
|
||||||
|
PARKINSON'S DISEASE |
||||||
|
|
||||||
|
|
PARKINSON'S DISEASE NEWS
|
|
||||
|
APRIL 2012
18th April 2012 - News release LEVODOPA-CARBIDOPA INTESTINAL GEL CLINICAL TRIAL RESULTS
Participants had had Parkinson's Disease for an average of over 10 years, and experienced an average of over 6 hours "off" time. Their average "off" time decreased by 4 hours per day with LCIG, which was 1.9 fewer hours of "off" time compared to levodopa-carbidopa IR in tablet form. Adverse events occurred in 95% of people on LCIG, and 100% of people on levodopa-carbidopa IR tablets. The most common adverse events were complication of device insertion (51%), abdominal pain (42%), procedural pain (32%), nausea (25%), constipation (21%), orthostatic hypotension (18%), post-operative wound infection (17%), and incision site erythema (16%). Treatment-related serious adverse events were reported in 14% of the LCIG patients, and 21% of the levodopa-carbidopa IR tablet patients. For more information go to the News release. For a printable version of this article click here. In order to refer to this article on its own click here.
9th April 2012 - New research THE COMMUNITY WITH THE WORST PREVALENCE OF PARKINSON'S DISEASE Neuroepidemiology [2012] 38 (3) : 154-163 (E.M.Khedr, G.S.Al Attar, M.R.Kandil, N.F.Kamel, N.Abo Elfetoh, M.A.Ahmed) Complete abstract Along the River Nile in Egypt has been found to have one of the world's highest prevalences of Parkinson's Disease. With a prevalence rate of 557 per 100,000 it makes Egypt the country with the world's second highest prevalence of Parkinson's Disease behind Albania. The greatest difference in the prevalence of Parkinson's Disease was between the illiterate and the literate, with a ratio of 3.93 to 1, especially in rural areas. This means that illiterate Egyptians in rural areas along the Nile south of Cairo are far more likely to develop Parkinson's Disease than any other community in the world, with a prevalence rate of 1,103 per 100,000. This difference is probably related to poverty rather than literacy. In some of the villages south of Cairo there are only mud roads and open sewers.
4th April 2012 - News release NEUPRO APPROVED FOR PARKINSON'S DISEASE The U.S. Food and Drug Administration (FDA) have approved the use of UCB's drug Neupro (rotigotine transdermal system) in the U.S.A. for the treatment of the signs and symptoms of advanced stage idiopathic Parkinson�s Disease and as a treatment for moderate-to-severe primary Restless Legs Syndrome (RLS). The FDA has also approved UCB�s new formulation of Neupro. Neupro will be available in U.S. retail pharmacies in July 2012. For more information go to the News release.
|
||||||
.gif) |
||||||
| �2006-2012 Viartis | ||||||
| [email protected] | ||||||
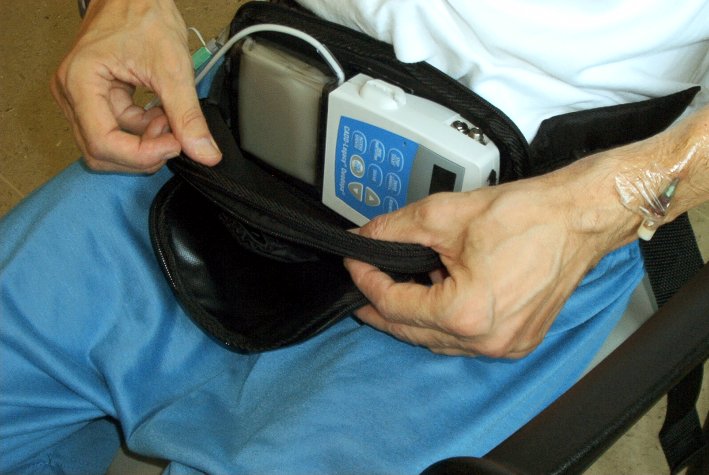 Abbott�s
have announced the results of the Phase 3 clinical trial in the U.S.A. of their
investigational treatment for advanced Parkinson�s Disease. The treatment
involved levodopa-carbidopa intestinal gel (LCIG). The treatment is already
approved in many countries outside the U.S.A. as Duodopa. LCIG contains the same
active medication as Sinemet, which is levodopa-carbidopa IR tablets. LCIG was
administered using a procedurally-implanted tube connected to a portable pump
that delivers the medication directly into the small intestine, where it is
absorbed into the bloodstream, providing a continuous delivery of medication
during the 16 hours a day of pump use.
Abbott�s
have announced the results of the Phase 3 clinical trial in the U.S.A. of their
investigational treatment for advanced Parkinson�s Disease. The treatment
involved levodopa-carbidopa intestinal gel (LCIG). The treatment is already
approved in many countries outside the U.S.A. as Duodopa. LCIG contains the same
active medication as Sinemet, which is levodopa-carbidopa IR tablets. LCIG was
administered using a procedurally-implanted tube connected to a portable pump
that delivers the medication directly into the small intestine, where it is
absorbed into the bloodstream, providing a continuous delivery of medication
during the 16 hours a day of pump use.  Amongst
those in their seventies, more than 7% of Egyptians were found to have
Parkinson's Disease. There were far more people in rural areas with
Parkinson's Disease, with a ratio of 3.2 to 1. Consistent with most other
countries, there were more men than women with Parkinson's Disease, with a male
female ratio of 1.7 to 1. Those with Parkinson's Disease were characterized by a
high prevalence of mood disorders, cognition dysfunction and gastrointestinal
symptoms. For more information go to the
Amongst
those in their seventies, more than 7% of Egyptians were found to have
Parkinson's Disease. There were far more people in rural areas with
Parkinson's Disease, with a ratio of 3.2 to 1. Consistent with most other
countries, there were more men than women with Parkinson's Disease, with a male
female ratio of 1.7 to 1. Those with Parkinson's Disease were characterized by a
high prevalence of mood disorders, cognition dysfunction and gastrointestinal
symptoms. For more information go to the
 In
April 2008, Neupro was withdrawn from use in the U.S.A. because specific batches
of Neupro had deviated from their specification. In June 2009, UCB proposed new
refrigerated storage conditions to alleviate crystallization on the patches. UCB
made progress in reformulation and remained committed to bringing Neupro to U.S.
patients. For more information go to the
In
April 2008, Neupro was withdrawn from use in the U.S.A. because specific batches
of Neupro had deviated from their specification. In June 2009, UCB proposed new
refrigerated storage conditions to alleviate crystallization on the patches. UCB
made progress in reformulation and remained committed to bringing Neupro to U.S.
patients. For more information go to the