26th July
2012 - News release
SINGLE DRUG FOR PARKINSON'S, ALZHEIMER'S AND MULTIPLE
SCLEROSIS
A new class of drug is being developed at
Northwestern University as a single therapy for Alzheimer�s Disease, Parkinson�s
Disease, Multiple Sclerosis and traumatic brain injuries by reducing
inflammation in the brain. Northwestern University has been issued patents to
cover this new drug class and has licensed the commercial development to a
biotech company that has recently completed the first human Phase 1 clinical
trial for the drug. The drugs in this class, represented by MW151 and MW189,
target a particular type of brain inflammation, which they claim is a common
denominator in these neurological disorders, as well as in traumatic brain
injury and stroke. They claim that brain inflammation plays a major role in the
progressive damage characteristic of these chronic diseases and brain injury.
For more information, go to the
News release.
 Despite
their claims, there is no evidence that inflammation is a primary cause of
Parkinson's Disease. Parkinson's Disease has been shown to be due to the
insufficient formation of dopamine, which is completely unaccounted for by this
method. Their recent study solely concerned Alzheimer's Disease in mice who did
not even have Alzheimer's Disease, and was not shown to rid or reduce
Alzheimer's Disease. They have not obtained any evidence at all concerning
efficacy in Parkinson's Disease. For a printable version of this article
click here.
In order to refer to this article on its own
click here.
Despite
their claims, there is no evidence that inflammation is a primary cause of
Parkinson's Disease. Parkinson's Disease has been shown to be due to the
insufficient formation of dopamine, which is completely unaccounted for by this
method. Their recent study solely concerned Alzheimer's Disease in mice who did
not even have Alzheimer's Disease, and was not shown to rid or reduce
Alzheimer's Disease. They have not obtained any evidence at all concerning
efficacy in Parkinson's Disease. For a printable version of this article
click here.
In order to refer to this article on its own
click here.
16th July
2012 - News release
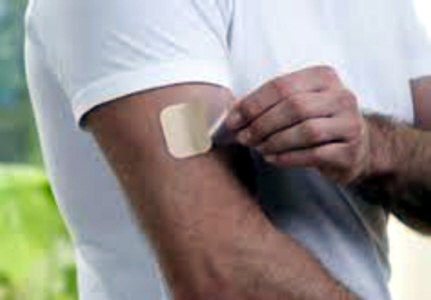
NEUPRO LAUNCHED IN THE U.S.A. TO TREAT PARKINSON'S DISEASE
The role of UCB announced today that Neupro (Rotigotine
Transdermal System) is now available in U.S. pharmacies. Neupro was approved by
the U.S Food and Drug Administration in April to treat the signs and symptoms of
early and advanced stage idiopathic Parkinson�s disease and moderate-to-severe
primary Restless Legs Syndrome. Neupro is a once-daily patch that provides
continuous delivery of the dopamine agonist rotigotine for 24 hours. Neupro is
available in four different dosage strengths for the signs and symptoms of
Parkinson�s disease (2 mg/24 hours, 4 mg/24 hours, 6 mg/24 hours, and 8 mg/24
hours).
 In
clinical trials, the most common adverse reactions were nausea, vomiting,
somnolence, application site reactions, dizziness, anorexia, insomnia,
hyperhidrosis, peripheral edema, and dyskinesia. There is an increased risk for
hallucinations in people with advanced-stage Parkinson�s Disease when they are treated
with Neupro. Neupro may cause symptomatic postural/orthostatic hypotension and
syncope (fainting), especially during dose escalation, elevated blood pressure,
elevated heart rate, weight gain and fluid retention. Neupro should be used with
caution in people with cardiovascular disease.
In
clinical trials, the most common adverse reactions were nausea, vomiting,
somnolence, application site reactions, dizziness, anorexia, insomnia,
hyperhidrosis, peripheral edema, and dyskinesia. There is an increased risk for
hallucinations in people with advanced-stage Parkinson�s Disease when they are treated
with Neupro. Neupro may cause symptomatic postural/orthostatic hypotension and
syncope (fainting), especially during dose escalation, elevated blood pressure,
elevated heart rate, weight gain and fluid retention. Neupro should be used with
caution in people with cardiovascular disease.
Case reports suggest that patients can experience
intense urges to gambling, increased sexual urges, intense urges to spend money,
binge eating, and other intense urges, and the inability to control these urges
while taking medications including Neupro. For more information, go to the
News release.
For a printable version of this article
click here.
In order to refer to this article on its own
click here.
13th July
2012 - New research
THE EFFECT OF PHYSIOTHERAPY ON PARKINSON'S DISEASE
Cochrane Database Systematic Reviews [2012] 7 : CD002817 (Tomlinson CL, Patel S,
Meek C, Clarke CE, Stowe R, Shah L, Sackley CM, Deane KH, Herd CP, Wheatley K,
Ives N.)
Complete abstract
The role of physiotherapy in Parkinson's Disease
aims to maximise functional ability and minimise secondary complications through
movement rehabilitation. However, there are many methods of physiotherapy that
have been used in Parkinson's Disease with differing effects. An analysis was
carried out of all the published studies concerning the use of physiotherapy in
Parkinson's Disease. Trials were classified into the following intervention
comparisons : general physiotherapy, exercise, treadmill training, cueing, dance
and martial arts
 Physiotherapy
was found to be beneficial using most methods in the short term (less than three
months). The benefit was significant when using the following tests : velocity,
step length, two-minute or six-minute walk tests, Timed Up & Go, Functional
Reach Test, Berg Balance Scale and clinician-rated UPDRS. However, for some
outcomes (velocity, Berg Balance Scale and UPDRS), the differences observed were
at, or approaching, what are considered minimally clinical important changes.
The review illustrates that a wide range of approaches are employed by
physiotherapists to treat Parkinson's Disease. There was no evidence of differences in treatment effect between the types of
physiotherapy used. For a printable version of this article
click here.
In order to refer to this article on its own
click here.
Physiotherapy
was found to be beneficial using most methods in the short term (less than three
months). The benefit was significant when using the following tests : velocity,
step length, two-minute or six-minute walk tests, Timed Up & Go, Functional
Reach Test, Berg Balance Scale and clinician-rated UPDRS. However, for some
outcomes (velocity, Berg Balance Scale and UPDRS), the differences observed were
at, or approaching, what are considered minimally clinical important changes.
The review illustrates that a wide range of approaches are employed by
physiotherapists to treat Parkinson's Disease. There was no evidence of differences in treatment effect between the types of
physiotherapy used. For a printable version of this article
click here.
In order to refer to this article on its own
click here.
8th July 2012 - New book
RATING SCALES IN PARKINSON'S DISEASE : CLINICAL PRACTICE
AND RESEARCH
Cristina Sampaio, Christopher G.Goetz, Anette Schrag
 Publisher's
description : For many years, the need to develop valid tools to evaluate signs
and symptoms of Parkinson Disease has been present. Rating Scales in Parkinson's
Disease: Clinical Practice and Research is written for researchers from the
medical and social sciences, and for health professionals wishing to evaluate
the progress of their patients suffering from Parkinson's Disease. The book is
both exhaustive in the description of the scales and informative on the
advantages and limitations of each scale. As such, the text clearly guides
readers on how to choose and use the instruments available. Extensive
cross-referenced tables and charts integrate the parts of the book to
facilitate readers in moving from one symptom domain to another.
Click
here for more details. For
more books concerning Parkinson's Disease go to
Parkinson's Disease Books.
In order to refer to this article on its own
click here.
Publisher's
description : For many years, the need to develop valid tools to evaluate signs
and symptoms of Parkinson Disease has been present. Rating Scales in Parkinson's
Disease: Clinical Practice and Research is written for researchers from the
medical and social sciences, and for health professionals wishing to evaluate
the progress of their patients suffering from Parkinson's Disease. The book is
both exhaustive in the description of the scales and informative on the
advantages and limitations of each scale. As such, the text clearly guides
readers on how to choose and use the instruments available. Extensive
cross-referenced tables and charts integrate the parts of the book to
facilitate readers in moving from one symptom domain to another.
Click
here for more details. For
more books concerning Parkinson's Disease go to
Parkinson's Disease Books.
In order to refer to this article on its own
click here.
1st July 2012 - New research
FIPAMEZOLE FOR DYSKINESIA IN PARKINSON'S DISEASE
Neurology 2012 Jun 27 [Epub ahead of print] (Lewitt PA, Hauser RA, Lu M,
Nicholas AP, Weiner W, Coppard N, Leinonen M, Savola JM.) Complete abstract
Fipamezole is a new a(2)-adrenergic receptor antagonist being assessed for its
use in treating the dyskinesia that can occur in Parkinson's Disease. Dyskinesia
is abnormal physical movements, in the form of writhing or jerky movements, that
can be caused by chronic use of L-dopa. Over half of people taking L-dopa can
eventually be affected by dyskinesia. The following video shows mild to moderate
dyskinesia
Video. The following video shows severe
dyskinesia
Video.
 The
study was carried out in the U.S.A. and India. The total study population showed
no statistically significant difference. However, because of the differences
between the U.S. and Indian study populations, a prespecified subgroup
analysis of U.S. subjects was conducted, showing fipamezole at 90 mg moderately
reduced dyskinesia that was due to to L-dopa. The response was shown to be
according to the dose used when assessing the different dosages (30mg, 60mg, 90
mg fipamezole). Fipamezole induced mild transient blood pressure elevation and
was associated with "an acceptable profile of adverse effects." For a printable version of this article
click here. In order to refer to this
article on its own
click here.
The
study was carried out in the U.S.A. and India. The total study population showed
no statistically significant difference. However, because of the differences
between the U.S. and Indian study populations, a prespecified subgroup
analysis of U.S. subjects was conducted, showing fipamezole at 90 mg moderately
reduced dyskinesia that was due to to L-dopa. The response was shown to be
according to the dose used when assessing the different dosages (30mg, 60mg, 90
mg fipamezole). Fipamezole induced mild transient blood pressure elevation and
was associated with "an acceptable profile of adverse effects." For a printable version of this article
click here. In order to refer to this
article on its own
click here.
.gif)
.gif)
 Despite
their claims, there is no evidence that inflammation is a primary cause of
Parkinson's Disease. Parkinson's Disease has been shown to be due to the
insufficient formation of dopamine, which is completely unaccounted for by this
method. Their recent study solely concerned Alzheimer's Disease in mice who did
not even have Alzheimer's Disease, and was not shown to rid or reduce
Alzheimer's Disease. They have not obtained any evidence at all concerning
efficacy in Parkinson's Disease. For a printable version of this article
Despite
their claims, there is no evidence that inflammation is a primary cause of
Parkinson's Disease. Parkinson's Disease has been shown to be due to the
insufficient formation of dopamine, which is completely unaccounted for by this
method. Their recent study solely concerned Alzheimer's Disease in mice who did
not even have Alzheimer's Disease, and was not shown to rid or reduce
Alzheimer's Disease. They have not obtained any evidence at all concerning
efficacy in Parkinson's Disease. For a printable version of this article
 In
clinical trials, the most common adverse reactions were nausea, vomiting,
somnolence, application site reactions, dizziness, anorexia, insomnia,
hyperhidrosis, peripheral edema, and dyskinesia. There is an increased risk for
hallucinations in people with advanced-stage Parkinson�s Disease when they are treated
with Neupro. Neupro may cause symptomatic postural/orthostatic hypotension and
syncope (fainting), especially during dose escalation, elevated blood pressure,
elevated heart rate, weight gain and fluid retention. Neupro should be used with
caution in people with cardiovascular disease.
In
clinical trials, the most common adverse reactions were nausea, vomiting,
somnolence, application site reactions, dizziness, anorexia, insomnia,
hyperhidrosis, peripheral edema, and dyskinesia. There is an increased risk for
hallucinations in people with advanced-stage Parkinson�s Disease when they are treated
with Neupro. Neupro may cause symptomatic postural/orthostatic hypotension and
syncope (fainting), especially during dose escalation, elevated blood pressure,
elevated heart rate, weight gain and fluid retention. Neupro should be used with
caution in people with cardiovascular disease. Physiotherapy
was found to be beneficial using most methods in the short term (less than three
months). The benefit was significant when using the following tests : velocity,
step length, two-minute or six-minute walk tests, Timed Up & Go, Functional
Reach Test, Berg Balance Scale and clinician-rated UPDRS. However, for some
outcomes (velocity, Berg Balance Scale and UPDRS), the differences observed were
at, or approaching, what are considered minimally clinical important changes.
The review illustrates that a wide range of approaches are employed by
physiotherapists to treat Parkinson's Disease. There was no evidence of differences in treatment effect between the types of
physiotherapy used. For a printable version of this article
Physiotherapy
was found to be beneficial using most methods in the short term (less than three
months). The benefit was significant when using the following tests : velocity,
step length, two-minute or six-minute walk tests, Timed Up & Go, Functional
Reach Test, Berg Balance Scale and clinician-rated UPDRS. However, for some
outcomes (velocity, Berg Balance Scale and UPDRS), the differences observed were
at, or approaching, what are considered minimally clinical important changes.
The review illustrates that a wide range of approaches are employed by
physiotherapists to treat Parkinson's Disease. There was no evidence of differences in treatment effect between the types of
physiotherapy used. For a printable version of this article
 Publisher's
description : For many years, the need to develop valid tools to evaluate signs
and symptoms of Parkinson Disease has been present. Rating Scales in Parkinson's
Disease: Clinical Practice and Research is written for researchers from the
medical and social sciences, and for health professionals wishing to evaluate
the progress of their patients suffering from Parkinson's Disease. The book is
both exhaustive in the description of the scales and informative on the
advantages and limitations of each scale. As such, the text clearly guides
readers on how to choose and use the instruments available. Extensive
cross-referenced tables and charts integrate the parts of the book to
facilitate readers in moving from one symptom domain to another.
Publisher's
description : For many years, the need to develop valid tools to evaluate signs
and symptoms of Parkinson Disease has been present. Rating Scales in Parkinson's
Disease: Clinical Practice and Research is written for researchers from the
medical and social sciences, and for health professionals wishing to evaluate
the progress of their patients suffering from Parkinson's Disease. The book is
both exhaustive in the description of the scales and informative on the
advantages and limitations of each scale. As such, the text clearly guides
readers on how to choose and use the instruments available. Extensive
cross-referenced tables and charts integrate the parts of the book to
facilitate readers in moving from one symptom domain to another.
 The
study was carried out in the U.S.A. and India. The total study population showed
no statistically significant difference. However, because of the differences
between the U.S. and Indian study populations, a prespecified subgroup
analysis of U.S. subjects was conducted, showing fipamezole at 90 mg moderately
reduced dyskinesia that was due to to L-dopa. The response was shown to be
according to the dose used when assessing the different dosages (30mg, 60mg, 90
mg fipamezole). Fipamezole induced mild transient blood pressure elevation and
was associated with "an acceptable profile of adverse effects." For a printable version of this article
The
study was carried out in the U.S.A. and India. The total study population showed
no statistically significant difference. However, because of the differences
between the U.S. and Indian study populations, a prespecified subgroup
analysis of U.S. subjects was conducted, showing fipamezole at 90 mg moderately
reduced dyskinesia that was due to to L-dopa. The response was shown to be
according to the dose used when assessing the different dosages (30mg, 60mg, 90
mg fipamezole). Fipamezole induced mild transient blood pressure elevation and
was associated with "an acceptable profile of adverse effects." For a printable version of this article