.gif) VIARTIS
|
||||||
|
PARKINSON'S DISEASE |
||||||
|
|
||||||
|
|
PARKINSON'S DISEASE NEWS
|
|
||||
|
DECEMBER 2011
18th December 2011 - News release PROSAVIN CLINICAL TRIAL RESULTS FOR PARKINSON'S DISEASE
The degree of efficacy is quite moderate and declines after six months. The average improvement in Parkinson's Disease symptoms for all the dosages was about 27% after 3 months. This improved slightly to about 31% after 6 months. The improvements started to decline after that down to 29% after 1 year, and declined further down to only 23% after 2 years. Three dosages were assessed : 1x, 2x and 5x. The level of efficacy declined when the higher 5x dosage was used. More results are expected in 2012 for the 5x dose. An enhanced administration procedure that facilitates higher dosing was used with some patients, but failed to demonstrate any additional benefit. The safety profile was described as being favourable with no serious adverse events, but details of the side effects were not provided. Oxford BioMedica have claimed that the method could potentially provide more than a 10-fold increase in dopamine formation. However, the moderate improvement in efficacy is entirely inconsistent with that suggestion. Although they have claimed that three genes and enzymes are required for dopamine formation, only two of them are actually needed. Stimulating gene and enzyme levels artificially as they are doing reduces a person's own formation of those genes and enzymes, which is probably why the results start to deteriorate after six months. For more information go to the News release. In order to refer to this article on its own click here.
15th December 2011 - New research INFLUENZA TREBLES THE RISK OF PARKINSON SYMPTOMS Influenza and other respiratory viruses [2011] 5 (5) : 328-333 (S.Toovey, S.S.Jick, C.R.Meier) Complete abstract Influenza has been found to treble the risk of Parkinson symptoms. Influenza has been associated with Encephalitis Lethargica, a medical disorder causing the symptoms of Parkinson's Disease following the 1918 influenza pandemic. For more information go to Encephalitis Lethargica.
10th December 2011 - New research DUODOPA WITH COMT INHIBITORS FOR PARKINSON'S DISEASE European Journal of Neurology [2011] Dec 5 [Epub ahead of print] (D.Nyholm, A.Johansson, H.Lennern�s, H. Askmark) Complete abstract
COMT inhibitors (Catechol-O-methyltransferase inhibitors) may be used to decrease the need for L-dopa, because they reduce its breakdown. COMT inhibitors have already been successfully used orally as Stalevo, which is a combination of L-dopa and carbidopa (the same as Sinemet), plus a COMT inhibitor (Entacapone). So researchers have investigated whether COMT inhibitors can also be taken orally in order to reduce the L-dopa requirement used with Duodopa. Both major COMT inhibitors (entacapone and tolcapone) were tested. The additional oral use of either of the COMT inhibitors was found to reduce the need of L-dopa by 20%. They did this without altering plasma L-dopa concentrations, reducing symptoms, or by reducing "off" time. In order to refer to this article on its own click here.
9th December 2011 - News report BOXING LEGEND DIAGNOSED WITH PARKINSON'S DISEASE
7th December 2011 - New research PRAMIPEXOLE (MIRAPEX) INCREASES THE RISK OF HEART FAILURE
Pharmacological Research [2011] Nov 23 [Epub ahead of print] (Mokhles
MM, Trifir� G, Dieleman JP, Haag MD, van Soest EM, Verhamme KM, Mazzaglia G,
Herings R, de Luise C, Ross D, Brusselle G, Colao A, Haverkamp W, Schade R, van
Camp G, Zanettini R, Sturkenboom MC.)
Complete abstract
2nd December 2011 - News release NEW INHALED VERSION OF L-DOPA The Michael J. Fox Foundation has awarded a grant to Civitas Therapeutics for clinical trials of CVT-301, which is a new inhaled version of L-dopa. It is claimed that CVT-301 has the potential to produce rapid, consistent and durable relief from the motor fluctuations associated with Parkinson�s Disease. Civitas has conducted a range of preclinical studies demonstrating CVT-301�s ability to deliver more rapid and consistent systemic exposure of L-dopa compared to the oral administration of L-dopa. The efficacy of oral L-dopa is significantly compromised by delayed absorption and excessive variability in the circulating drug concentrations. It is anticipated that the inhaled version of L-dopa would be used alongside the use of oral L-dopa. For more information go to the News release.
|
||||||
.gif) |
||||||
| �2006-2011 Viartis | ||||||
| [email protected] | ||||||
 Oxford BioMedica, a gene therapy company,
have announced updated clinical data from a Phase I/II clinical trial of
ProSavin for the treatment of Parkinson�s Disease.
ProSavin uses Oxford BioMedica's own
Oxford BioMedica, a gene therapy company,
have announced updated clinical data from a Phase I/II clinical trial of
ProSavin for the treatment of Parkinson�s Disease.
ProSavin uses Oxford BioMedica's own
 Recent
influenza (influenza in the past month) was associated
with a trebling of the likelihood of Parkinson symptoms.
Influenza some time in the past year was associated with only a small increase
in the likelihood of Parkinson symptoms. The number of previous attacks
progressively increased the likelihood of Parkinson symptoms. However, influenza
did not increase the likelihood of actual Parkinson's Disease. The relevance of
this research may be that people could be wrongly diagnosed with Parkinson's
Disease, when what they have actually had is influenza. Around 25% of
people are wrongly diagnosed with Parkinson's Disease and do not actually have
it. In order to refer to this article on
its own
Recent
influenza (influenza in the past month) was associated
with a trebling of the likelihood of Parkinson symptoms.
Influenza some time in the past year was associated with only a small increase
in the likelihood of Parkinson symptoms. The number of previous attacks
progressively increased the likelihood of Parkinson symptoms. However, influenza
did not increase the likelihood of actual Parkinson's Disease. The relevance of
this research may be that people could be wrongly diagnosed with Parkinson's
Disease, when what they have actually had is influenza. Around 25% of
people are wrongly diagnosed with Parkinson's Disease and do not actually have
it. In order to refer to this article on
its own 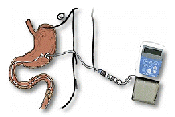 Duodopa
is a combination of L-dopa and carbidopa in the form of a gel. It is
administered throughout the day using a portable pump directly into the small
intestine through a surgically placed tube. This ensures a flow of L-dopa that
can be adjusted according to the person's needs. It enables more consistent
plasma concentrations of L-dopa. The side effects of Duodopa are similar
to those observed with oral administration of L-dopa and carbidopa. For more
information go to
Duodopa
is a combination of L-dopa and carbidopa in the form of a gel. It is
administered throughout the day using a portable pump directly into the small
intestine through a surgically placed tube. This ensures a flow of L-dopa that
can be adjusted according to the person's needs. It enables more consistent
plasma concentrations of L-dopa. The side effects of Duodopa are similar
to those observed with oral administration of L-dopa and carbidopa. For more
information go to
 The
former
world boxing champion, the Colombian Antonio Cervantes, has been
diagnosed with Parkinson's Disease. After Muhammad Ali he is the most successful
boxer ever to have Parkinson's Disease. Antonio Cervantes grew up in Colombia
shining shoes and selling contraband cigarettes in order to survive. He became a
professional boxer whilst still only 18. He became the World Light Welterweight
champion in 1972. He held the world title twice, from 1972 to 1976 and 1977
to 1980. He successfully defended his world title 16 times, and had a total of
21 world championship contests. He retired from boxing in 1983 having had 106
contests. For more information go to
The
former
world boxing champion, the Colombian Antonio Cervantes, has been
diagnosed with Parkinson's Disease. After Muhammad Ali he is the most successful
boxer ever to have Parkinson's Disease. Antonio Cervantes grew up in Colombia
shining shoes and selling contraband cigarettes in order to survive. He became a
professional boxer whilst still only 18. He became the World Light Welterweight
champion in 1972. He held the world title twice, from 1972 to 1976 and 1977
to 1980. He successfully defended his world title 16 times, and had a total of
21 world championship contests. He retired from boxing in 1983 having had 106
contests. For more information go to
 The
use of pramipexole by people with Parkinson's Disease has been found to increase
the risk of heart failure. Pramipexole is a dopamine agonist that is also
known as Mirapex, Mirapexin, or Sifrol. For more information go to
The
use of pramipexole by people with Parkinson's Disease has been found to increase
the risk of heart failure. Pramipexole is a dopamine agonist that is also
known as Mirapex, Mirapexin, or Sifrol. For more information go to
 CVT-301
uses the ARCUS inhalation technology, which delivers a reliable and
consistent drug dose with a compact, breath actuated inhaler. It uses a
proprietary dry powder and inhaler combination that is unique in its ability to
deliver a large, precise dose independent of inspiratory flow rate from a
simple, easy-to-use device suitable for convenient self-administration. The
platform has successfully delivered more than one million doses to patients
incorporating active agents ranging from small molecules to large proteins. In order to refer to this article on its own
CVT-301
uses the ARCUS inhalation technology, which delivers a reliable and
consistent drug dose with a compact, breath actuated inhaler. It uses a
proprietary dry powder and inhaler combination that is unique in its ability to
deliver a large, precise dose independent of inspiratory flow rate from a
simple, easy-to-use device suitable for convenient self-administration. The
platform has successfully delivered more than one million doses to patients
incorporating active agents ranging from small molecules to large proteins. In order to refer to this article on its own